Actor Portrayal
VIVIMUSTA is an alkylating drug indicated for the treatment of adult patients with:
Myelosuppression: VIVIMUSTA causes myelosuppression. Bendamustine hydrochloride (HCl) caused severe myelosuppression (Grade 3-4) in 98% of patients in the two NHL studies. Three patients (2%) died from myelosuppression-related adverse reactions; one each from neutropenic sepsis, diffuse alveolar hemorrhage with Grade 3 thrombocytopenia and pneumonia from an opportunistic infection (CMV).
Monitor complete blood counts, including leukocytes, platelets, hemoglobin (Hgb), and neutrophils frequently. In the clinical trials, blood counts were monitored every week initially. Hematologic nadirs were observed predominantly in the third week of therapy.
Myelosuppression may require dose delays and/or subsequent dose reductions if recovery to the recommended values has not occurred by the first day of the next scheduled cycle. Delay the next cycle of therapy if ANC less than 1 X 109/L OR PLATELET COUNT LESS THAN 75 X 109/L.
Infection, including pneumonia, sepsis, septic shock, hepatitis, and death has occurred in adult and pediatric patients in clinical trials and in postmarketing reports for bendamustine HCl. Patients with myelosuppression following treatment with bendamustine HCl are more susceptible to infections. Advise patients with myelosuppression following VIVIMUSTA treatment to contact a healthcare provider (HCP) immediately if they have symptoms or signs of infections.
Patients treated with VIVIMUSTA are at risk for reactivation of infections including (but not limited to) hepatitis B, cytomegalovirus, Mycobacterium tuberculosis, and herpes zoster. Implement appropriate measures for infection and infection reactivation prior to administration.
PML, including fatal cases, have occurred following treatment with bendamustine, primarily in combination with rituximab or obinutuzumab. Consider PML in the differential diagnosis in patients with new or worsening neurological, cognitive, or behavioral signs and symptoms. If PML is suspected, withhold VIVIMUSTA treatment and perform appropriate diagnostic evaluations. Consider discontinuation or reduction of any concomitant chemotherapy or immunosuppressive therapy in patients who develop PML.
Infusion-related reactions to bendamustine HCl have occurred commonly in clinical trials. Symptoms include fever, chills, pruritus, and rash. In rare instances, severe anaphylactic and anaphylactoid reactions have occurred, particularly in the second and subsequent cycles of therapy.
Monitor clinically and discontinue drug for severe reactions. Ask patients about symptoms suggestive of infusion-related reactions after their first cycle of therapy. Do not rechallenge patients who experienced Grade 3 or worse allergic-type reactions. Consider measures to prevent severe reactions in patients who have experienced Grade 1 or 2 infusion-related reactions. Discontinue for patients with Grade 4 infusion-related reactions and consider discontinuation for Grade 3 infusion-related reactions as clinically appropriate considering individual benefits, risks, and supportive care.
Tumor lysis syndrome associated with bendamustine HCl has occurred in patients in clinical trials and in postmarketing reports. The onset tends to be within the first treatment cycle of bendamustine HCl and without intervention, may lead to acute renal failure and death.
Administer vigorous hydration and monitor blood chemistry, particularly potassium and uric acid levels, at baseline and closely during treatment with VIVIMUSTA.
Fatal and serious skin reactions have been reported with bendamustine HCl treatment in clinical trials and postmarketing safety reports, including toxic skin reactions [Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS)] bullous exanthema, and rash. Events occurred when bendamustine HCl was given as a single agent and in combination with other anticancer agents or allopurinol. Where skin reactions occur, they may be progressive and increase in severity with further treatment.
Monitor patients with skin reactions closely. If skin reactions are severe or progressive, withhold or discontinue VIVIMUSTA.
Fatal and serious cases of liver injury have been reported with bendamustine HCl injections. Combination therapy, progressive disease or reactivation of hepatitis B were confounding factors in some patients. Most cases were reported within the first three months of starting therapy.
Monitor liver chemistry tests prior to and during treatment with VIVIMUSTA.
Pre-malignant and malignant disease have developed in patients who have been treated with bendamustine HCl, including myelodysplastic syndrome, myeloproliferative disorders, acute myeloid leukemia, bronchial carcinoma, and non-melanoma skin cancer including basal cell carcinoma and squamous cell carcinoma.
Bendamustine HCl extravasations have been reported in postmarketing resulting in hospitalizations from erythema, marked swelling, and pain.
Assure good venous access prior to starting VIVIMUSTA infusion and monitor the intravenous infusion site for redness, swelling, pain, infection, and necrosis during and after administration of VIVIMUSTA.
Based on findings from animal reproduction studies and the drug’s mechanism of action, VIVIMUSTA can cause fetal harm when administered to a pregnant woman. Single intraperitoneal doses of bendamustine (that approximated the maximum recommended human dose based on body surface area) to pregnant mice and rats during organogenesis caused adverse developmental outcomes, including an increase in resorptions, skeletal and visceral malformations, and decreased fetal body weights.
See Females and Males of Reproductive Potential.
Adverse reactions (>5%) during infusion and within 24 hours post-infusion are nausea, and fatigue.
Most common adverse reactions (≥15%) for CLL are anemia, thrombocytopenia, neutropenia, lymphopenia, leukopenia, hyperbilirubinemia, pyrexia, nausea, vomiting.
Most common adverse reactions (≥15%) for NHL are lymphopenia, leukopenia, anemia, neutropenia, thrombocytopenia, nausea, fatigue, vomiting, diarrhea, pyrexia, constipation, anorexia, cough, headache, weight decreased dyspnea, rash, and stomatitis.
These are not all the possible side effects of VIVIMUSTA. Please see Full Prescribing Information for a full list.
The coadministration of VIVIMUSTA with CYP1A2 inhibitors may increase bendamustine plasma concentrations and may result in increased incidence of adverse reactions with VIVIMUSTA. Consider alternative therapies that are not CYP1A2 inhibitors during treatment with VIVIMUSTA.
The coadministration of VIVIMUSTA with CYP1A2 inducers may decrease bendamustine plasma concentrations and may result in decreased efficacy of VIVIMUSTA. Consider alternative therapies that are not CYP1A2 inducers during treatment with VIVIMUSTA.
See full Prescribing Information for Specific Drugs and Interactions.
Advise pregnant women of the potential risk to a fetus.
Because of the potential for serious adverse reactions in the breastfed child, advise women not to breastfeed during treatment with VIVIMUSTA and for 1 week after the last dose.
VIVIMUSTA can cause embryo-fetal harm when administered to a pregnant woman. Advise female patients of reproductive potential to use effective contraception during treatment with VIVIMUSTA and for 6 months after the last dose. Advise men and female partners of reproductive potential to use effective contraception during treatment with VIVIMUSTA and for 3 months after the last dose.
Safety and effectiveness of VIVIMUSTA has not been established in pediatric patients.
Do not use VIVIMUSTA in patients with creatinine clearance (Clcr) less than 30 mL/min.
Do not use VIVIMUSTA in patients with AST or ALT 2.5 to 10 times upper limit of normal (ULN) and total bilirubin 1.5 to 3 times ULN or total bilirubin greater than 3 times ULN.
The Important Safety Information does not include all the information needed to use VIVIMUSTA safely and effectively. Please see accompanying full PDF Document: Prescribing Information (File Size: 441 KB) for VIVIMUSTA.
To Report SUSPECTED ADVERSE REACTIONS, contact Azurity Pharmaceuticals, Inc. at 1-800-461-7449, or FDA at 1-800-FDA-1088 or www.fda.gov/MedWatch.
Vivimusta® is a trademark of Azurity Pharmaceuticals, Inc.
© 2025 Azurity Pharmaceuticals, Inc.
PP-VIV-US-0168
0.9% sodium chloride injection USP or 2.5% dextrose/0.45% sodium chloride injection USP
(2˚C to 8˚C or 36˚F to 46˚F) or 3 hours when stored at room temperature (15˚C to 30˚C or 59˚F to 86˚F) and room light
Recommended Vivimusta® dosage is 100 mg/m2 administered intravenously
Recommended Vivimusta® dosage is 120 mg/m2 administered intravenously
Volume of Vivimusta® Required for Dilution Into 250 mL of 0.9% Sodium Chloride Injection, USP, or 2.5% Dextrose/0.45% Sodium Chloride Injection, USP for A Given Dose and Body Surface Area
| Body Surface Area (m2) | Volume of Vivimusta® to Withdraw (mL) From Vial | |||||
|---|---|---|---|---|---|---|
| 120 mg/m2 | 100 mg/m2 | 90 mg/m2 | 60 mg/m2 | 50 mg/m2 | 25 mg/m2 | |
| 1 | 4.8 | 4 | 3.6 | 2.4 | 2 | 1 |
| 1.1 | 5.3 | 4.4 | 4 | 2.6 | 2.2 | 1.1 |
| 1.2 | 5.8 | 4.8 | 4.3 | 2.9 | 2.4 | 1.2 |
| 1.3 | 6.2 | 5.2 | 4.7 | 3.1 | 2.6 | 1.3 |
| 1.4 | 6.7 | 5.6 | 5 | 3.4 | 2.8 | 1.4 |
| 1.5 | 7.2 | 6 | 5.4 | 3.6 | 3 | 1.5 |
| 1.6 | 7.7 | 6.4 | 5.8 | 3.8 | 3.2 | 1.6 |
| 1.7 | 8.2 | 6.8 | 6.1 | 4.1 | 3.4 | 1.7 |
| 1.8 | 8.6 | 7.2 | 6.5 | 4.3 | 3.6 | 1.8 |
| 1.9 | 9.1 | 7.6 | 6.8 | 4.6 | 3.8 | 1.9 |
| 2 | 9.6 | 8 | 7.2 | 4.8 | 4 | 2 |
| 2.1 | 10.1 | 8.4 | 7.6 | 5 | 4.2 | 2.1 |
| 2.2 | 10.6 | 8.8 | 7.9 | 5.3 | 4.4 | 2.2 |
| 2.3 | 11 | 9.2 | 8.3 | 5.5 | 4.6 | 2.3 |
| 2.4 | 11.5 | 9.6 | 8.6 | 5.8 | 4.8 | 2.4 |
| 2.5 | 12 | 10 | 9 | 6 | 5 | 2.5 |
| 2.6 | 12.5 | 10.4 | 9.4 | 6.2 | 5.2 | 2.6 |
| 2.7 | 13 | 10.8 | 9.7 | 6.5 | 5.4 | 2.7 |
| 2.8 | 13.4 | 11.2 | 10.1 | 6.7 | 5.6 | 2.8 |
| 2.9 | 13.9 | 11.6 | 10.4 | 7 | 5.8 | 2.9 |
| 3 | 14.4 | 12 | 10.8 | 7.2 | 6 | 3 |
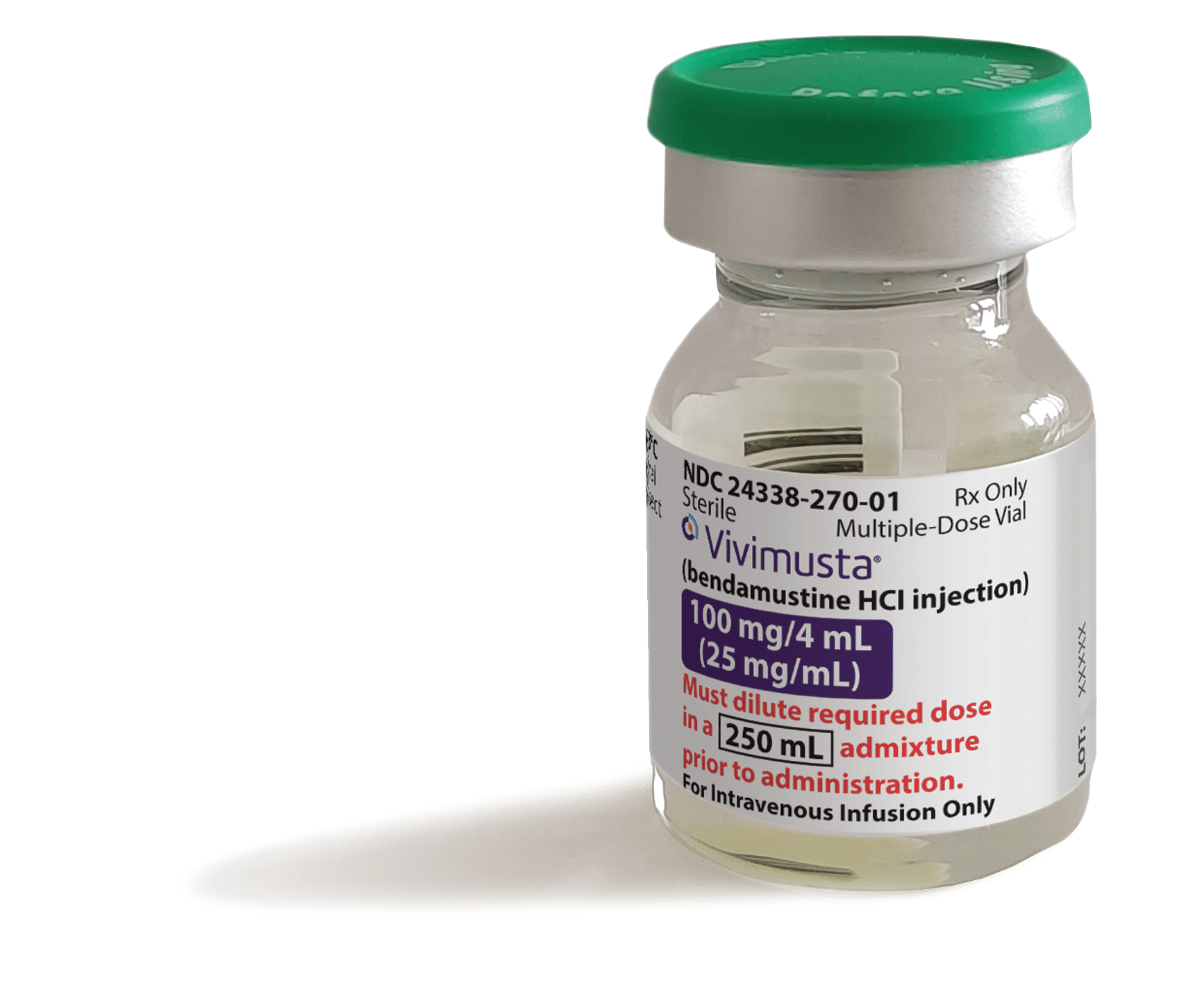
Product labeling, packaging, and imagery are for representation purposes only and shall constitute the property of Azurity.
24338-270-01
J9056 Injection, bendamustine hydrochloride
(Vivimusta®), 1 mg
96413 Chemotherapy administration, intravenous infusion technique; up to 1 hour, single or initial substance/drug
P: 1-800-746-6273
P: 1-800-633-7555
P: 1-877-453-3972
P: 1-877-625-2566
P: 1-800-482-6700
P: 1-800-304-3064
P: 1-800-710-6100
VIVIMUSTA is an alkylating drug indicated for the treatment of adult patients with:
Myelosuppression: VIVIMUSTA causes myelosuppression. Bendamustine hydrochloride (HCl) caused severe myelosuppression (Grade 3-4) in 98% of patients in the two NHL studies. Three patients (2%) died from myelosuppression-related adverse reactions; one each from neutropenic sepsis, diffuse alveolar hemorrhage with Grade 3 thrombocytopenia and pneumonia from an opportunistic infection (CMV).
Monitor complete blood counts, including leukocytes, platelets, hemoglobin (Hgb), and neutrophils frequently. In the clinical trials, blood counts were monitored every week initially. Hematologic nadirs were observed predominantly in the third week of therapy.
Myelosuppression may require dose delays and/or subsequent dose reductions if recovery to the recommended values has not occurred by the first day of the next scheduled cycle. Delay the next cycle of therapy if ANC less than 1 X 109/L OR PLATELET COUNT LESS THAN 75 X 109/L.
Infections: Infection, including pneumonia, sepsis, septic shock, hepatitis, and death has occurred in adult and pediatric patients in clinical trials and in postmarketing reports for bendamustine HCl. Patients with myelosuppression following treatment with bendamustine HCl are more susceptible to infections. Advise patients with myelosuppression following VIVIMUSTA treatment to contact a healthcare provider (HCP) immediately if they have symptoms or signs of infections.
Patients treated with VIVIMUSTA are at risk for reactivation of infections including (but not limited to) hepatitis B, cytomegalovirus, Mycobacterium tuberculosis, and herpes zoster. Implement appropriate measures for infection and infection reactivation prior to administration.
Progressive multifocal leukoencephalopathy (PML): PML, including fatal cases, have occurred following treatment with bendamustine, primarily in combination with rituximab or obinutuzumab. Consider PML in the differential diagnosis in patients with new or worsening neurological, cognitive, or behavioral signs and symptoms. If PML is suspected, withhold VIVIMUSTA treatment and perform appropriate diagnostic evaluations. Consider discontinuation or reduction of any concomitant chemotherapy or immunosuppressive therapy in patients who develop PML.
Anaphylaxis and Infusion-Related Reactions: Infusion-related reactions to bendamustine HCl have occurred commonly in clinical trials. Symptoms include fever, chills, pruritus, and rash. In rare instances, severe anaphylactic and anaphylactoid reactions have occurred, particularly in the second and subsequent cycles of therapy.
Monitor clinically and discontinue drug for severe reactions. Ask patients about symptoms suggestive of infusion-related reactions after their first cycle of therapy. Do not rechallenge patients who experienced Grade 3 or worse allergic-type reactions. Consider measures to prevent severe reactions in patients who have experienced Grade 1 or 2 infusion-related reactions. Discontinue for patients with Grade 4 infusion-related reactions and consider discontinuation for Grade 3 infusion-related reactions as clinically appropriate considering individual benefits, risks, and supportive care.
Tumor Lysis Syndrome: Tumor lysis syndrome associated with bendamustine HCl has occurred in patients in clinical trials and in postmarketing reports. The onset tends to be within the first treatment cycle of bendamustine HCl and without intervention, may lead to acute renal failure and death.
Administer vigorous hydration and monitor blood chemistry, particularly potassium and uric acid levels, at baseline and closely during treatment with VIVIMUSTA.
Skin Reactions: Fatal and serious skin reactions have been reported with bendamustine HCl treatment in clinical trials and postmarketing safety reports, including toxic skin reactions [Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS)] bullous exanthema, and rash. Events occurred when bendamustine HCl was given as a single agent and in combination with other anticancer agents or allopurinol. Where skin reactions occur, they may be progressive and increase in severity with further treatment.
Monitor patients with skin reactions closely. If skin reactions are severe or progressive, withhold or discontinue VIVIMUSTA.
Hepatotoxicity: Fatal and serious cases of liver injury have been reported with bendamustine HCl injections. Combination therapy, progressive disease or reactivation of hepatitis B were confounding factors in some patients. Most cases were reported within the first three months of starting therapy.
Monitor liver chemistry tests prior to and during treatment with VIVIMUSTA.
Other Malignancies: Pre-malignant and malignant disease have developed in patients who have been treated with bendamustine HCl, including myelodysplastic syndrome, myeloproliferative disorders, acute myeloid leukemia, bronchial carcinoma, and non-melanoma skin cancer including basal cell carcinoma and squamous cell carcinoma.
Extravasation Injury: Bendamustine HCl extravasations have been reported in postmarketing resulting in hospitalizations from erythema, marked swelling, and pain.
Assure good venous access prior to starting VIVIMUSTA infusion and monitor the intravenous infusion site for redness, swelling, pain, infection, and necrosis during and after administration of VIVIMUSTA.
Embryo-Fetal Toxicity: Based on findings from animal reproduction studies and the drug’s mechanism of action, VIVIMUSTA can cause fetal harm when administered to a pregnant woman. Single intraperitoneal doses of bendamustine (that approximated the maximum recommended human dose based on body surface area) to pregnant mice and rats during organogenesis caused adverse developmental outcomes, including an increase in resorptions, skeletal and visceral malformations, and decreased fetal body weights.
See Females and Males of Reproductive Potential.
Adverse reactions (>5%) during infusion and within 24 hours post-infusion are nausea, and fatigue.
Most common adverse reactions (≥15%) for CLL are anemia, thrombocytopenia, neutropenia, lymphopenia, leukopenia, hyperbilirubinemia, pyrexia, nausea, vomiting.
Most common adverse reactions (≥15%) for NHL are lymphopenia, leukopenia, anemia, neutropenia, thrombocytopenia, nausea, fatigue, vomiting, diarrhea, pyrexia, constipation, anorexia, cough, headache, weight decreased dyspnea, rash, and stomatitis.
These are not all the possible side effects of VIVIMUSTA. Please see Full Prescribing Information for a full list.
CYP1A2 Inhibitors
The coadministration of VIVIMUSTA with CYP1A2 inhibitors may increase bendamustine plasma concentrations and may result in increased incidence of adverse reactions with VIVIMUSTA. Consider alternative therapies that are not CYP1A2 inhibitors during treatment with VIVIMUSTA.
CYP1A2 Inducers
The coadministration of VIVIMUSTA with CYP1A2 inducers may decrease bendamustine plasma concentrations and may result in decreased efficacy of VIVIMUSTA. Consider alternative therapies that are not CYP1A2 inducers during treatment with VIVIMUSTA.
See full Prescribing Information for Specific Drugs and Interactions.
Pregnancy: Advise pregnant women of the potential risk to a fetus.
Lactation: Because of the potential for serious adverse reactions in the breastfed child, advise women not to breastfeed during treatment with VIVIMUSTA and for 1 week after the last dose.
Females and Males of Reproductive Potential: VIVIMUSTA can cause embryo-fetal harm when administered to a pregnant woman. Advise female patients of reproductive potential to use effective contraception during treatment with VIVIMUSTA and for 6 months after the last dose. Advise men and female partners of reproductive potential to use effective contraception during treatment with VIVIMUSTA and for 3 months after the last dose.
Pediatric Patients: Safety and effectiveness of VIVIMUSTA has not been established in pediatric patients.
Renal Impairment: Do not use VIVIMUSTA in patients with creatinine clearance (Clcr) less than 30 mL/min.
Hepatic Impairment: Do not use VIVIMUSTA in patients with AST or ALT 2.5 to 10 times upper limit of normal (ULN) and total bilirubin 1.5 to 3 times ULN or total bilirubin greater than 3 times ULN.
The Important Safety Information does not include all the information needed to use VIVIMUSTA safely and effectively. Please see accompanying full Prescribing Information for VIVIMUSTA.
To Report SUSPECTED ADVERSE REACTIONS, contact Azurity Pharmaceuticals, Inc. at 1-800-461-7449, or FDA at 1-800-FDA-1088 or www.fda.gov/MedWatch.
VIVIMUSTA® is a trademark of Azurity Pharmaceuticals, Inc.
© 2025 Azurity Pharmaceuticals, Inc.
PP-VIV-US-0168
Reference: Vivimusta [package insert], Woburn, MA: Azurity Pharmaceuticals, Inc.; 2025
Please confirm that you are a US healthcare professional.